


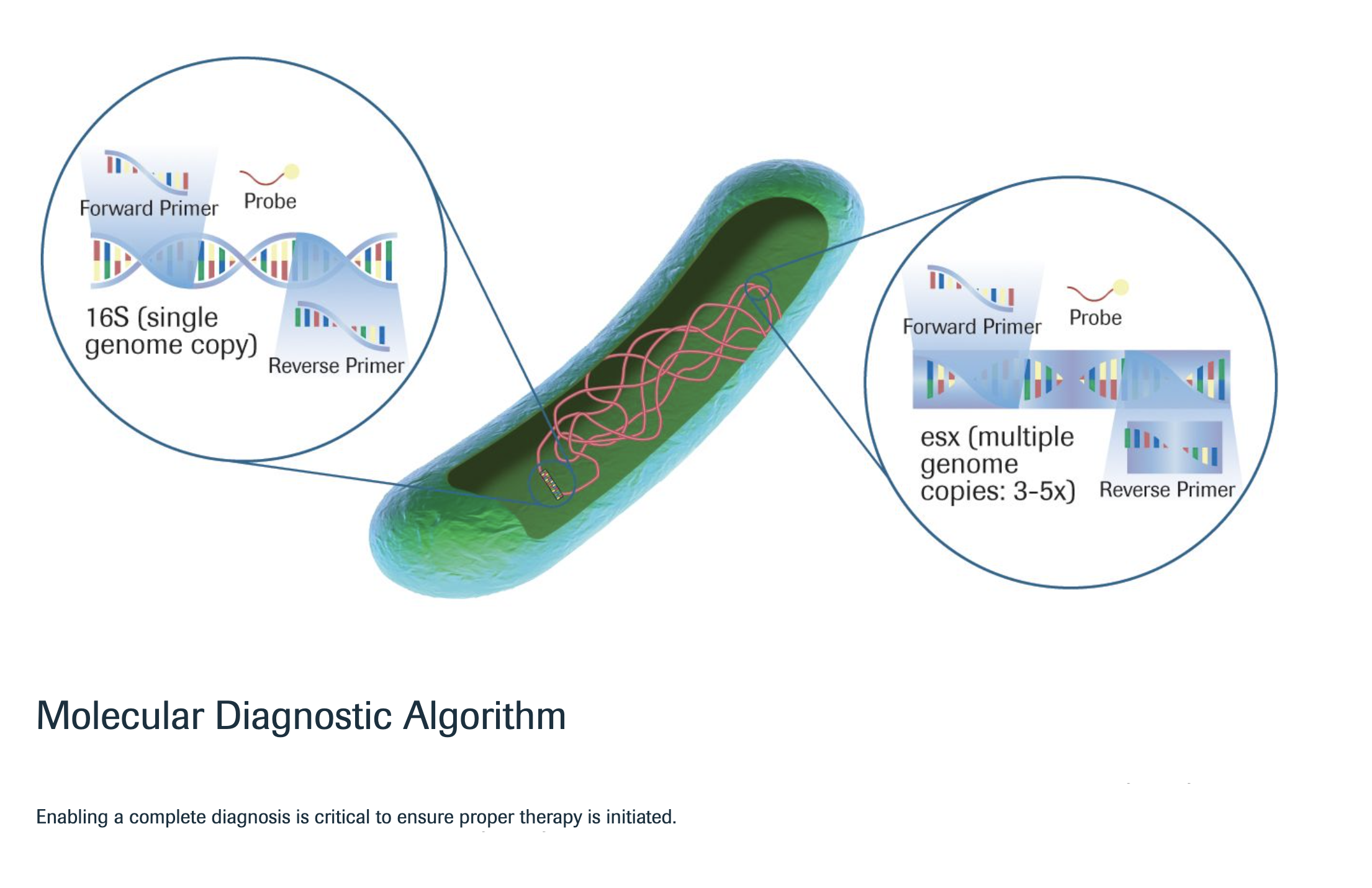

Format: 100 rxns/Kit (Pack of 50 Kits)
27,400$






Format: 100 rxns/Kit (Pack of 50 Kits)

| crb® Real-Time PCR MTBC/NTM kit is a real-time molecular diagnostic test for the screening and differentiation of Mycobacterium tuberculosis complex and atypical mycobacteria/ nontuberculous mycobacteria extracted from clinical samples |

| SKU | MD021 |
| Detection |
|
| Specimen |
|
| Kit Content |
|
| Compatible Instrument | Real-time PCR instrument |
| Test per kit | 100 |